Àdàpọ̀ kẹ́míkà
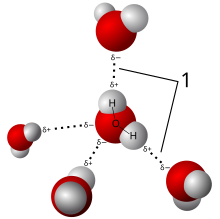
Àdàpọ̀ kẹ́míkà jẹ́ is a pure ohun kẹ́míkà ògidì kan tó ní ẹ́límẹ̀ntì kẹ́míkà méjì tàbí jùbẹ́ẹ̀lọ ọ̀tọ̀ọ̀tọ̀[1][2][3] tó ṣe é yàsọ́tọ̀ sí ohun míràn pẹ̀lú ìdarapọ̀ kẹ́míkà.[4] Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms[3] that are held together in a defined spatial arrangement by chemical bonds. Chemical compounds can be molecular compounds held together by covalent bonds, salts held together by ionic bonds, intermetallic compounds held together by metallic bonds, or complexes held together by coordinate covalent bonds. Pure chemical elements are not considered chemical compounds, even if they consist of molecules that contain only multiple atoms of a single element (such as H2, S8, etc.),[5] which are called diatomic molecules or polyatomic molecules.

|
Àyọkà yìí tàbí apá rẹ̀ únfẹ́ àtúnṣe sí. Ẹ le fẹ̀ jù báyìí lọ tàbí kí ẹ ṣàtúnṣe rẹ̀ lọ́nà tí yíò mu kúnrẹ́rẹ́. Ẹ ran Wikipedia lọ́wọ́ láti fẹ̀ẹ́ jù báyìí lọ. |
Itokasi
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]- ↑ Brown, Theodore L.; LeMay, H. Eugene; Bursten, Bruce E.; Murphy, Catherine J.; Woodward, Patrick (2009), Chemistry: The Central Science, AP Edition (11th ed.), Upper Saddle River, NJ: Prentice Hall, pp. 5–6, ISBN 0-13-236489-1
- ↑ Hill, John W.; Petrucci, Ralph H.; McCreary, Terry W.; Perry, Scott S. (2005), General Chemistry (4th ed.), Upper Saddle River, NJ: Prentice Hall, p. 6, ISBN 978-0-13-140283-6
- ↑ 3.0 3.1 Whitten, Kenneth W.; Davis, Raymond E.; Peck, M. Larry (2000), General Chemistry (6th ed.), Fort Worth, TX: Saunders College Publishing/Harcourt College Publishers, p. 15, ISBN 978-0-03-072373-5
- ↑ Wilbraham, Antony; Matta, Michael; Staley, Dennis; Waterman, Edward (2002), Chemistry (1st ed.), Upper Saddle River, NJ: Prentice Hall, p. 36, ISBN 0-13-251210-6
- ↑ Halal, John (2008), "Chapter 8: General Chemistry" (PDF), Milady's Hair Structure and Chemistry Simplified (5 ed.), Milady Publishing, pp. 96–98, ISBN 1-4283-3558-7
