Klorídì alumíníọ́mù
Ìrísí
(Àtúnjúwe láti Aluminium chloride)
| Klorídì alumíníọ́mù | |
|---|---|

| |
| |
klorídì alumíníọ́mù | |
| Àwọn orúkọ mìíràn | klorídì alumíníọ́mù(III) |
| Identifiers | |
| CAS number | 7446-70-0 (anhydrous), 10124-27-3 (hydrate), 7784-13-6 (hexahydrate) |
| PubChem | 24012 |
| ChEBI | CHEBI:30114 |
| nọ́mbà RTECS | BD0530000 |
| ATC code | D10 |
| SMILES | Cl[Al](Cl)Cl
|
| InChI | 1/Al.3ClH/h;3*1H/q+3;;;/p-3
|
| InChI key | VSCWAEJMTAWNJL-DFZHHIFOAR |
| ChemSpider ID | 22445 |
| Properties | |
| Molecular formula | AlCl3 |
| Molar mass | 133.34 g/mol (anhydrous) 241.43 g/mol (hexahydrate) |
| Appearance | white or pale yellow solid, hygroscopic |
| Density | 2.48 g/cm3 (anhydrous) 1.3 g/cm3 (hexahydrate) |
| Ojúàmì ìyọ́ |
192.4 °C *(anhydrous) |
| Ojúàmì ìhó |
120 °C (hexahydrate) |
| Solubility in water | 43.9 g/100 ml (0 °C) 44.9 g/100 ml (10 °C) 45.8 g/100 ml (20 °C) 46.6 g/100 ml (30 °C) 47.3 g/100 ml (40 °C) 48.1 g/100 ml (60 °C) 48.6 g/100 ml (80 °C) 49 g/100 ml (100 °C) |
| Solubility | soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride slightly soluble in benzene |
| Structure | |
| Crystal structure | Monoclinic, mS16 |
| Space group | C12/m1, No. 12 |
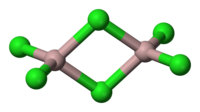
| Jẹ́ómẹ́trì ìjọfẹnukò |
Octahedral (solid) Tetrahedral (liquid) |
| Molecular shape | Trigonal planar (monomeric vapour) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−704 kJ·mol−1[1] |
| Standard molar entropy S |
111 J·mol−1·K−1[1] |
| Hazards | |
| EU classification | Corrosive (C) |
| R-phrases | R34 |
| S-phrases | (S1/2), S7/8, S28, S45 |
| LD50 | anhydrous: 380 mg/kg, rat (oral) hexahydrate: 3311 mg/kg, rat (oral) |
| Related compounds | |
| Other anions | Aluminium fluoride Aluminium bromide Aluminium iodide |
| Other cations | Boron trichloride Gallium trichloride Indium(III) chloride Magnesium chloride |
| Related Lewis acids | Iron(III) chloride Boron trifluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Klorídì alumíníọ́mù (AlCl3) ni àdàpọ̀ pàtàkì ti alumíníọ́mù àti klorínì. Funfun ni àwọ̀ rẹ̀, sùgbọ́n ó sábà ní ìdọ̀tí triklorídì irin tó únsọ àwọ̀ rẹ̀ di pípọ́n. Tó bá jẹ́ adirapọ̀, ó ní ojúàmì ìyọ́ ati ìhó kúkúrú. Níbi tí wọ́n ti únṣe ẹ̀ṣọ́ alumíníọ́mù ni wọ́n ti únsábà ṣe é àti lò ó, sùgbọ́n ó tún wúlò ní àwọn ilẹ́-iṣẹ́ kẹ́míkà míràn. Wọ́n sábà tọ́ka sí àdàpọ̀ yìí bíi ásìdì Lewis. Ó jẹ́ àpẹrẹ àdàpọ̀ àìníọ́rgánì tó "únfọ́" ní tẹ́mprétọ̀ tó lọ́wọ́rọ́, láti yípadà sẹ́yìn láti polymer sí mólékùlù.
Itokasi
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]- ↑ 1.0 1.1 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed.. Houghton Mifflin Company. ISBN 0-618-94690-X.
