Baeyer–Emmerling indole synthesis
Ìrísí
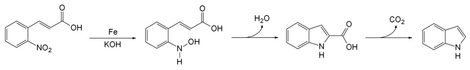
Baeyer–Emmerling indole synthesis jẹ́ ọ̀nà fún ìṣètò indole lati (ìrọ́pò) ortho-nitrocinnamic acid àti iron gbẹrẹfu nínú àpòpọ̀ base tó le.[1][2] Ẹni tí ó ṣe àwàrí ìdàpapọ̀ ṣiṣẹ́ yìí ni Adolf von Baeyer àti Adolph Emmerling ní ọdún 1869.[3] [4]

Ètò ìdàpọ̀ ṣiṣẹ́
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]Ìdàpọ̀ ṣiṣẹ́ iron gbẹrẹfu pẹ̀lú o-nitrocinnamic mú ẹgbẹ́ nito kù sí nitroso. Nitrogen yìí maa dìpọ̀ pẹ̀lú carbon lórí okùn alkene pẹ̀lú sísọ molecule omi kan nù lati di ohun róbótó. Yíyọ ẹgbẹ́ carbonyl kúro ma a jẹ́ kí indole jẹyọ.
Tún wo
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]Àwọn ìtọ́kasí
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]- ↑ Bayer, A.; Emmerling, A. (1869). "Synthese des indoles". Berichte der deutschen chemischen Gesellschaft 2 (1): 679–682. doi:10.1002/cber.186900201268.
- ↑ Baeyer 5 Archived 2007-08-16 at the Wayback Machine..
- ↑ Chamberlain, Joseph Scudder (1921). A Textbook of Organic Chemistry. Blakiston. p. 874. http://books.google.com/books?id=9ls6AAAAMAAJ&pg=PA874.
- ↑ Lockyer, Sir Norman (1881). "Indigo and its Artificial Production". Nature 24 (610): 227. doi:10.1038/024227c0. http://books.google.com/books?id=TMMKAAAAYAAJ&pg=PA229.
