DNA
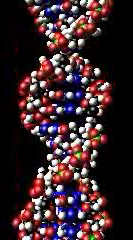
DNA duro fun Deoxyribonucleic acid ni ede geesi, eyi tumosi deoksiribonúkléì kíkan (DNA tabi DNK). DNA je núkléì kíkan ti o ni awon ilana abimo ti a n lo fun idagbasoke olounalàyè gbogbo awon iru ahamo emi. Lamolara, DNA je alolara eleemeji. Awon apa DNA to ungbe awon ilana wonyi ni a unpe ni abimo, sugbon awon itelentele DNA miran wa fun idimule, tabi won wa fun iseeseese ilo ilana abimo yi. Lapapo mo RNA ati awon amoralokun, DNA je ikan ninu awon horogbangba pataki meta to se koko fun gbogbo irun ohun elemi.
DNA ni awon alarapupo gigun meji awon eyo kekere kan ton je nukleotidi, pelu igbaeyin to je adipo suga ati oniyofosforu ti won je sisopapao pelu awon ide esteri. Awon atinrin mejeji yi jo doju de ona odi si ara won bi be won je olodiajodojuko. Awon ihun jije lilemo suga kookan ni ikan ninu awon iru horo merin tounje ipilenukleu (lasan bi, awon ipile). Itelentele awon ipilenukleu mererin yi leba igbaeyin ni won un samioro iwifun. Iwifun yi je kika pelu lilo amioro alabimo, to utokasi itelentele awon kikan amino ninu awon proteini. Amioro na je kika nipa sise àwòkọ awon ìnà DNA si inu kikan nukleiki RNA to baramu nnu igbese kan to unje isawoko.
Ninu awon ahamo DNA je gbigbajo si idimu gigun kan to unje kromosomu. Nigba ipin ahamo awon kromosomu yi je didameji ninu igbese itunda DNA, to si unpese fun ahamo kookan awon kromosomu pipe tikookan fun won. Awon agbarajo eukarioti (awon eranko, ogbin, ehu, ati protisti) ko opo awon DNA won pamo sinu kóróonú àhámọ́ ati awon DNA won miran sinu awon apainu àhámọ́, bi mitokondria tabi adáláwọ̀.[1] Lafiwe si awon prokarioti (bakteria ati arkea) ti won unko DNA won pamo sinu idanuahamo nikan. Ninu awon kromosomu, awo proteini kromatini bi histoni undipo o si unsegbajo DNA. Awon idimu idipo yi unselana ibasepo larin DNA ati awon proteini miran, nigba tounsejanu awon apa DNA wo ni yio sawoko.
Awon ohun ini
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]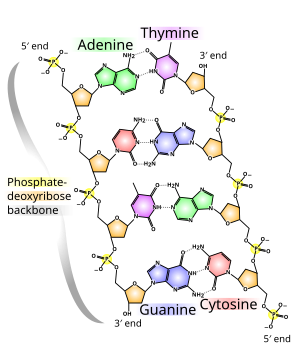
DNA je is a long alarapupo gigun to je dida lati awo eyo to un tunde to nje nukleotidi.[2][3][4] Bo se koko je wiwari latowo James D. Watson ati Francis Crick, idimu DNA gbogbo awon irueda ni awon ewon onilopo meji ti ikookan won lo ipo kanna ka, ti ikookan won si ni ite 34 Ångström (3.4 nanometres) ati itanka 10 Ångström (1.0 nanometres).[5] Gegebi àgbékà miran se so, nigba to je wiwon nidu omiadalu pato kan, ewon DNA ni iwon 22 de 26 Ångström ni fife (2.2 to 2.6 nanometres), be sini eyo nukleotidi kan ni iwon 3.3 Å (0.33 nm) ni gigun.[6] Botilejepe ikookan eyo to untunde je kenkele, awon alarapupo DNA le je horo titobi to ni egbeegberun nukleotidi ninu. Fun apere, kromosomu omoniyan to tobijulo, kromosomu nomba 1, je bi 220 egbeegberun ipile meji ni gigun.[7]
Ninu awon agbarajo alaye DNA ki saba wa bi horo kan soso, sugbon bi awon horo meji ti won je didimupo ni lilelile.[5][8] Awon mejeji lorapo bi eka igi, won ri bi alopo emeji. Awon nukleotidi atun ni apa igbaeyin horo, to mu ẹ̀wọ̀n na papo, ati ipilenukleu kan, to se basepo mo atinrin DNA miran ninu alopo na. Ipilenukleu kan to so po mo suga kan lo nje nukleosidi be sini ipile kan to so mo suga kan ati mo ikan tabi opo adipo oniyofosforu lo nje nukleotidi. Awon alarapupo ti won ni opo nukleotidi ti wo so po mo ara won (bi tinu DNA) lo nje nukleotidipupo.[9]
Igbaeyin atinrin DNA je dida lati ibi awon ìṣẹ́kù oniyofosforu ati suga.[10] Suga inu DNA ni 2-deoksiribosi, to je suga pentosi (karbonu-marun). Awon suga na je sisopo latowo awon idipo oniyofosforu ti won da awon ìdè fosfodiesteri larin awon s between the third and fifth carbon atomu karbonu keta ati ikarun awon oruka suga itosi. Awon ide alaidogba yi tumosi pe atinrin DNA ni idojude. Ninu alopo emeji idojude awon nukleotidi ninu atinrin kan kojusi idojude won ninu atinrin keji: awon atinrin yi je the strands are olodiajodojuko. Awon enu alaidogba awon atinrin DNA lo nje enu 5′ (arun akoko/five prime) ati 3′ (eta akoko/three prime), pelu enu 5' to pari pelu adipo oniyofosforu ati enu 3' to pari pelu adipo haidroksi. Iyato kan pataki t wa larin DNA ati RNA ni iru suga ti won ni, DNA ni suga pentosi 2-deoksiribosi nigbati RNA ni suga pentosi ribosi.[8]
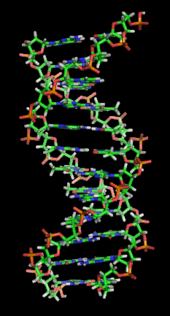
Alopo emeji DNA double helix je gbigbero pelu ipa meji: awon ide haidrojin larin awon nukleotidi ati awon ibasepo ipele ipile larin awon ipilenukleu oloorundidun.[12] Ninu ayika olomi ahamo, awon ide π adarapo awon ipile nukleotidi naro mo ipo horo DNA, láti dín iye ìbáṣepọ̀ wọn pọ̀ mọ́ igbá ìṣèdàlú kù ati bíi bẹ́ẹ̀, okun òmìnira Gibbs. Àwọn ìpìlẹ̀ mẹ́rẹ̀ẹ̀rin tó wà nínú DNA ni adẹnínì (kíkékúrú sí A), sitosínì (kíkékúrú sí C), guanínì (kíkékúrú sí G) àti timínì (kíkékúrú sí T). Àwọn ìpìlẹ̀ mẹ́rẹ̀ẹ̀rin yìí ni wọ́n so pọ̀ mọ́ ṣúgà/oníyọ̀fósfórù láti dá odidi núkléótídì, bó ṣe hàn fún adenosínì oníyọ̀fósfórùkan.
Àwọn ìpìlẹ̀núkléù pín sí irú méjì: àwọn purínì, A àti G, tí wọ́n jẹ́ ọlọ́mọ ẹgbẹ́ márùún àti mẹ́fà àwọn àdàpọ̀ aláàyípoọ̀tọ̀ọ̀tọ̀, àti àwọn pirimidínì, ọlọ́mọ ẹgbẹ́ mẹ́fà C àti T.[8] Ìpìlẹ̀núkléù pirimidínì karùún, urasílì (kíkékúrú sí U), ló úndípò timínì nínú RNA, ó sì yàtọ̀ sí timínì nkpa pé kò ní àdìpọ̀ mẹ́tílì nínú òrùka rẹ̀. Urasílì kì í sábà sí nínú DNA, ọ́ únṣẹlẹ̀ nìkan bíi èso ìfọ́wẹ́wẹ́ sitosínì. Bakanna mọ́ RNA àti DNA iye nínlá àwọn a large number of artificial núkléì kíkan ajọra aláfọwọ́dá náà tún ti jẹ́ dídá láti le ṣe àgbékà àwọn ohun ìní núkléì kíkan, tàbí fún lílò nínú ọ̀rọ̀oníṣẹ́ọ̀nàalàyè.[13]
Àwọn ibitooro
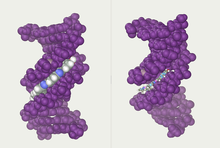
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]Àwọn atínrín alọ́po méjéèjì náà dá igbáẹ̀yìn DNA. Alọ́po ẹ̀mẹjì míràn kan náà tún ṣe é rí nípa títẹ̀lé àwọn àyè, tàbi àwọn ibitooro, tó wà láàrin àwọn atínrín náà. Àwọn àyè yìí wà nítòsí sí àwọn ìpìlẹ̀ méjì ó sìl lè pèsè ojú ìdè. Nítorípé àwọn atínrín kò wà níwájú ara wọn, àwọn ibitooro lò ní ìtóbi dídọ́gba. Ibitooro kan, ibitooro àgbà, jẹ́ 22 Å ní fífẹ̀, èkejì, ibitooro kékeré, jẹ́ 12 Å ní fífẹ̀.[14] Híhá ibitooro kékeré túmọ̀sí pé àwọn etí àwọn ìpìlẹ̀ ṣe é bọ́sí nínú ibitooro àgbà. Nítorí bẹ́ẹ̀, àwọn proteínì bíi àwọn akópa ìṣàwòkọ tí wọ́n le so mọ́ àwọn ìtẹ̀léntẹ̀lé pàtó nínú DNA alátínrín méjì únsábà farakan àwọn ẹ̀gbẹ́ àwọn ìpìlẹ̀ tí wọ́n hàn nínú ibitooro àgbà.[15] This situation varies in unusual conformations of DNA within the cell (see below), but the major and minor grooves are always named to reflect the differences in size that would be seen if the DNA is twisted back into the ordinary B form.
Ìṣeméjì ìpílẹ̀
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]In a DNA double helix, each type of nucleobase on one strand normally interacts with just one type of nucleobase on the other strand. This is called complementary base pairing. Here, purines form hydrogen bonds to pyrimidines, with A bonding only to T, and C bonding only to G. This arrangement of two nucleotides binding together across the double helix is called a base pair. As hydrogen bonds are not covalent, they can be broken and rejoined relatively easily. The two strands of DNA in a double helix can therefore be pulled apart like a zipper, either by a mechanical force or high temperature.[16] As a result of this complementarity, all the information in the double-stranded sequence of a DNA helix is duplicated on each strand, which is vital in DNA replication. Indeed, this reversible and specific interaction between complementary base pairs is critical for all the functions of DNA in living organisms.[3]

|

|
The two types of base pairs form different numbers of hydrogen bonds, AT forming two hydrogen bonds, and GC forming three hydrogen bonds (see figures, left). DNA with high GC-content is more stable than DNA with low GC-content. Although it is often stated that this is due to the added stability of an additional hydrogen bond, this is incorrect.Àdàkọ:Source? DNA with high GC-content is more stable due to intra-strand base stacking interactions.Àdàkọ:Cn
As noted above, most DNA molecules are actually two polymer strands, bound together in a helical fashion by noncovalent bonds; this double stranded structure (dsDNA) is maintained largely by the intrastrand base stacking interactions, which are strongest for G,C stacks. The two strands can come apart – a process known as melting – to form two ss DNA molecules. Melting occurs when conditions favor ssDNA; such conditions are high temperature, low salt and high pH (low pH also melts DNA, but since DNA is unstable due to acid depurination, low pH is rarely used). The stability of the dsDNA form depends not only on the GC-content (% G,C basepairs) but also on sequence (since stacking is sequence specific) and also length (longer molecules are more stable). The stability can be measured in various ways; a common way is the "melting temperature", which is the temperature at which 50% of the ds molecules are converted to ss molecules; melting temperature is dependent on ionic strength and the concentration of DNA. As a result, it is both the percentage of GC base pairs and the overall length of a DNA double helix that determine the strength of the association between the two strands of DNA. Long DNA helices with a high GC-content have stronger-interacting strands, while short helices with high AT content have weaker-interacting strands.[17] In biology, parts of the DNA double helix that need to separate easily, such as the TATAAT Pribnow box in some promoters, tend to have a high AT content, making the strands easier to pull apart.[18]
In the laboratory, the strength of this interaction can be measured by finding the temperature required to break the hydrogen bonds, their melting temperature (also called Tm value). When all the base pairs in a DNA double helix melt, the strands separate and exist in solution as two entirely independent molecules. These single-stranded DNA molecules (ssDNA) have no single common shape, but some conformations are more stable than others.[19]
Ọlọ́gbọ́n àti òdì-ọlọ́gbọ́n
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]A DNA sequence is called "sense" if its sequence is the same as that of a messenger RNA copy that is translated into protein.[20] The sequence on the opposite strand is called the "antisense" sequence. Both sense and antisense sequences can exist on different parts of the same strand of DNA (i.e. both strands contain both sense and antisense sequences). In both prokaryotes and eukaryotes, antisense RNA sequences are produced, but the functions of these RNAs are not entirely clear.[21] One proposal is that antisense RNAs are involved in regulating gene expression through RNA-RNA base pairing.[22]
A few DNA sequences in prokaryotes and eukaryotes, and more in plasmids and viruses, blur the distinction between sense and antisense strands by having overlapping genes.[23] In these cases, some DNA sequences do double duty, encoding one protein when read along one strand, and a second protein when read in the opposite direction along the other strand. In bacteria, this overlap may be involved in the regulation of gene transcription,[24] while in viruses, overlapping genes increase the amount of information that can be encoded within the small viral genome.[25]
Ìlọ́pogidi
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]DNA can be twisted like a rope in a process called DNA supercoiling. With DNA in its "relaxed" state, a strand usually circles the axis of the double helix once every 10.4 base pairs, but if the DNA is twisted the strands become more tightly or more loosely wound.[26] If the DNA is twisted in the direction of the helix, this is positive supercoiling, and the bases are held more tightly together. If they are twisted in the opposite direction, this is negative supercoiling, and the bases come apart more easily. In nature, most DNA has slight negative supercoiling that is introduced by enzymes called topoisomerases.[27] These enzymes are also needed to relieve the twisting stresses introduced into DNA strands during processes such as transcription and DNA replication.[28]

Àwọn ìdìmú DNA míràn
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]DNA exists in many possible conformations that include A-DNA, B-DNA, and Z-DNA forms, although, only B-DNA and Z-DNA have been directly observed in functional organisms.[10] The conformation that DNA adopts depends on the hydration level, DNA sequence, the amount and direction of supercoiling, chemical modifications of the bases, the type and concentration of metal ions, as well as the presence of polyamines in solution.[29]
The first published reports of A-DNA X-ray diffraction patterns— and also B-DNA used analyses based on Patterson transforms that provided only a limited amount of structural information for oriented fibers of DNA.[30][31] An alternate analysis was then proposed by Wilkins et al., in 1953, for the in vivo B-DNA X-ray diffraction/scattering patterns of highly hydrated DNA fibers in terms of squares of Bessel functions.[32] In the same journal, James D. Watson and Francis Crick presented their molecular modeling analysis of the DNA X-ray diffraction patterns to suggest that the structure was a double-helix.[5]
Although the `B-DNA form' is most common under the conditions found in cells,[33] it is not a well-defined conformation but a family of related DNA conformations[34] that occur at the high hydration levels present in living cells. Their corresponding X-ray diffraction and scattering patterns are characteristic of molecular paracrystals with a significant degree of disorder.[35][36]
Compared to B-DNA, the A-DNA form is a wider right-handed spiral, with a shallow, wide minor groove and a narrower, deeper major groove. The A form occurs under non-physiological conditions in partially dehydrated samples of DNA, while in the cell it may be produced in hybrid pairings of DNA and RNA strands, as well as in enzyme-DNA complexes.[37][38] Segments of DNA where the bases have been chemically modified by methylation may undergo a larger change in conformation and adopt the Z form. Here, the strands turn about the helical axis in a left-handed spiral, the opposite of the more common B form.[39] These unusual structures can be recognized by specific Z-DNA binding proteins and may be involved in the regulation of transcription.[40]
Ìsiṣẹ́olòógùn DNA míràn
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]For a number of years exobiologists have proposed the existence of a shadow biosphere, a postulated microbial biosphere of Earth that uses radically different biochemical and molecular processes than currently known life. One of the proposals was the existence of lifeforms that use arsenic instead of phosphorus in DNA.
A December 2010 NASA press conference stated that the bacterium GFAJ-1, which has evolved in an arsenic-rich environment, is the first terrestrial lifeform found which may have this ability. The bacterium was found in Mono Lake, east of Yosemite National Park. GFAJ-1 is a rod-shaped extremophile bacterium in the family Halomonadaceae that, when starved of phosphorus, may be capable of incorporating the usually poisonous element arsenic in its DNA.[41] This discovery may lend weight to the long-standing idea that extraterrestrial life could have a different chemical makeup from life on Earth.[41][42] The research was carried out by a team led by Felisa Wolfe-Simon, a geomicrobiologist and geobiochemist, a Postdoctoral Fellow of the NASA Astrobiology Institute with Arizona State University. This finding has, however, faced strong criticism from the scientific community; scientists have argued that there is no evidence that arsenic is actually incorporated into biomolecules.[42][43] Independent conformation of this finding has also not yet been possible.
Àwọn ìdìmú Quadruplex
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]At the ends of the linear chromosomes are specialized regions of DNA called telomeres. The main function of these regions is to allow the cell to replicate chromosome ends using the enzyme telomerase, as the enzymes that normally replicate DNA cannot copy the extreme 3′ ends of chromosomes.[44] These specialized chromosome caps also help protect the DNA ends, and stop the DNA repair systems in the cell from treating them as damage to be corrected.[45] In human cells, telomeres are usually lengths of single-stranded DNA containing several thousand repeats of a simple TTAGGG sequence.[46]

These guanine-rich sequences may stabilize chromosome ends by forming structures of stacked sets of four-base units, rather than the usual base pairs found in other DNA molecules. Here, four guanine bases form a flat plate and these flat four-base units then stack on top of each other, to form a stable G-quadruplex structure.[48] These structures are stabilized by hydrogen bonding between the edges of the bases and chelation of a metal ion in the centre of each four-base unit.[49] Other structures can also be formed, with the central set of four bases coming from either a single strand folded around the bases, or several different parallel strands, each contributing one base to the central structure.
In addition to these stacked structures, telomeres also form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle stabilized by telomere-binding proteins.[50] At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA and base pairing to one of the two strands. This triple-stranded structure is called a displacement loop or D-loop.[48]

|

|
| Single branch | Multiple branches |
DNA ẹlẹ́ka
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]In DNA fraying occurs when non-complementary regions exist at the end of an otherwise complementary double-strand of DNA. However, branched DNA can occur if a third strand of DNA is introduced and contains adjoining regions able to hybridize with the frayed regions of the pre-existing double-strand. Although the simplest example of branched DNA involves only three strands of DNA, complexes involving additional strands and multiple branches are also possible.[51] Branched DNA can be used in nanotechnology to construct geometric shapes, see the section on uses in technology below.
Ìgbọ̀ntìtì
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]DNA may carry out low-frequency collective motion as observed by the Raman spectroscopy[52][53] and analyzed with a quasi-continuum model.[54][55]

|
Àyọkà yìí tàbí apá rẹ̀ únfẹ́ àtúnṣe sí. Ẹ le fẹ̀ jù báyìí lọ tàbí kí ẹ ṣàtúnṣe rẹ̀ lọ́nà tí yíò mu kúnrẹ́rẹ́. Ẹ ran Wikipedia lọ́wọ́ láti fẹ̀ẹ́ jù báyìí lọ. |
Itokasi
[àtúnṣe | àtúnṣe àmìọ̀rọ̀]- ↑ Russell, Peter (2001). iGenetics. New York: Benjamin Cummings. ISBN 0-8053-4553-1.
- ↑ Saenger, Wolfram (1984). Principles of Nucleic Acid Structure. New York: Springer-Verlag. ISBN 0-387-90762-9.
- ↑ 3.0 3.1 Alberts, Bruce; Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts and Peter Walters (2002). Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 0-8153-3218-1. OCLC 48122761 57023651 69932405 145080076 48122761 57023651 69932405. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=mboc4.TOC&depth=2.
- ↑ Butler, John M. (2001). Forensic DNA Typing. Elsevier. ISBN 978-0-12-147951-0. OCLC 45406517 223032110 45406517. pp. 14–15.
- ↑ 5.0 5.1 5.2 Watson J.D. and Crick F.H.C. (1953). "A Structure for Deoxyribose Nucleic Acid" (PDF). Nature 171 (4356): 737–738. Bibcode 1953Natur.171..737W. doi:10.1038/171737a0. PMID 13054692. http://www.nature.com/nature/dna50/watsoncrick.pdf.
- ↑ Mandelkern M, Elias J, Eden D, Crothers D (1981). "The dimensions of DNA in solution". J Mol Biol 152 (1): 153–61. doi:10.1016/0022-2836(81)90099-1. PMID 7338906.
- ↑ Gregory S; Barlow, KF; McLay, KE; Kaul, R; Swarbreck, D; Dunham, A; Scott, CE; Howe, KL et al. (2006). "The DNA sequence and biological annotation of human chromosome 1". Nature 441 (7091): 315–21. Bibcode 2006Natur.441..315G. doi:10.1038/nature04727. PMID 16710414.
- ↑ 8.0 8.1 8.2 Berg J., Tymoczko J. and Stryer L. (2002) Biochemistry. W. H. Freeman and Company ISBN 0-7167-4955-6
- ↑ Abbreviations and Symbols for Nucleic Acids, Polynucleotides and their Constituents IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Retrieved 03 January 2006.
- ↑ 10.0 10.1 Ghosh A, Bansal M (2003). "A glossary of DNA structures from A to Z". Acta Crystallogr D 59 (4): 620–6. doi:10.1107/S0907444903003251. PMID 12657780.
- ↑ Created from PDB 1D65
- ↑ Yakovchuk P, Protozanova E, Frank-Kamenetskii MD (2006). "Base-stacking and base-pairing contributions into thermal stability of the DNA double helix". Nucleic Acids Res. 34 (2): 564–74. doi:10.1093/nar/gkj454. PMC 1360284. PMID 16449200. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1360284.
- ↑ Verma S, Eckstein F (1998). "Modified oligonucleotides: synthesis and strategy for users". Annu. Rev. Biochem. 67: 99–134. doi:10.1146/annurev.biochem.67.1.99. PMID 9759484.
- ↑ Wing R, Drew H, Takano T, Broka C, Tanaka S, Itakura K, Dickerson R (1980). "Crystal structure analysis of a complete turn of B-DNA". Nature 287 (5784): 755–8. Bibcode 1980Natur.287..755W. doi:10.1038/287755a0. PMID 7432492.
- ↑ Pabo C, Sauer R (1984). "Protein-DNA recognition". Annu Rev Biochem 53: 293–321. doi:10.1146/annurev.bi.53.070184.001453. PMID 6236744.
- ↑ Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub H (2000). "Mechanical stability of single DNA molecules". Biophys J 78 (4): 1997–2007. Bibcode 2000BpJ....78.1997C. doi:10.1016/S0006-3495(00)76747-6. PMC 1300792. PMID 10733978. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1300792.
- ↑ Chalikian T, Völker J, Plum G, Breslauer K (1999). "A more unified picture for the thermodynamics of nucleic acid duplex melting: A characterization by calorimetric and volumetric techniques". Proc Natl Acad Sci USA 96 (14): 7853–8. Bibcode 1999PNAS...96.7853C. doi:10.1073/pnas.96.14.7853. PMC 22151. PMID 10393911. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=22151.
- ↑ deHaseth P, Helmann J (1995). "Open complex formation by Escherichia coli RNA polymerase: the mechanism of polymerase-induced strand separation of double helical DNA". Mol Microbiol 16 (5): 817–24. doi:10.1111/j.1365-2958.1995.tb02309.x. PMID 7476180.
- ↑ Isaksson J, Acharya S, Barman J, Cheruku P, Chattopadhyaya J (2004). "Single-stranded adenine-rich DNA and RNA retain structural characteristics of their respective double-stranded conformations and show directional differences in stacking pattern". Biochemistry 43 (51): 15996–6010. doi:10.1021/bi048221v. PMID 15609994.
- ↑ Designation of the two strands of DNA JCBN/NC-IUB Newsletter 1989, Accessed 07 May 2008
- ↑ Hüttenhofer A, Schattner P, Polacek N (2005). "Non-coding RNAs: hope or hype?". Trends Genet 21 (5): 289–97. doi:10.1016/j.tig.2005.03.007. PMID 15851066.
- ↑ Munroe S (2004). "Diversity of antisense regulation in eukaryotes: multiple mechanisms, emerging patterns". J Cell Biochem 93 (4): 664–71. doi:10.1002/jcb.20252. PMID 15389973.
- ↑ Makalowska I, Lin C, Makalowski W (2005). "Overlapping genes in vertebrate genomes". Comput Biol Chem 29 (1): 1–12. doi:10.1016/j.compbiolchem.2004.12.006. PMID 15680581.
- ↑ Johnson Z, Chisholm S (2004). "Properties of overlapping genes are conserved across microbial genomes". Genome Res 14 (11): 2268–72. doi:10.1101/gr.2433104. PMC 525685. PMID 15520290. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=525685.
- ↑ Lamb R, Horvath C (1991). "Diversity of coding strategies in influenza viruses". Trends Genet 7 (8): 261–6. PMID 1771674.
- ↑ Benham C, Mielke S (2005). "DNA mechanics". Annu Rev Biomed Eng 7: 21–53. doi:10.1146/annurev.bioeng.6.062403.132016. PMID 16004565.
- ↑ Champoux J (2001). "DNA topoisomerases: structure, function, and mechanism". Annu Rev Biochem 70: 369–413. doi:10.1146/annurev.biochem.70.1.369. PMID 11395412.
- ↑ Wang J (2002). "Cellular roles of DNA topoisomerases: a molecular perspective". Nat Rev Mol Cell Biol 3 (6): 430–40. doi:10.1038/nrm831. PMID 12042765.
- ↑ Basu H, Feuerstein B, Zarling D, Shafer R, Marton L (1988). "Recognition of Z-RNA and Z-DNA determinants by polyamines in solution: experimental and theoretical studies". J Biomol Struct Dyn 6 (2): 299–309. PMID 2482766.
- ↑ Franklin RE, Gosling RG (6 March 1953). "The Structure of Sodium Thymonucleate Fibres I. The Influence of Water Content". Acta Crystallogr 6 (8–9): 673–7. doi:10.1107/S0365110X53001939. Archived from the original on 14 June 2010. https://web.archive.org/web/20100614152932/http://hekto.med.unc.edu:8080/CARTER/carter_WWW/Bioch_134/PDF_files/Franklin_Gossling.pdf. Retrieved 1 December 2011.
Franklin RE, Gosling RG (1953). "The structure of sodium thymonucleate fibres. II. The cylindrically symmetrical Patterson function". Acta Crystallogr 6 (8–9): 678–85. doi:10.1107/S0365110X53001940. - ↑ Franklin, Rosalind and Gosling, Raymond (1953). "Molecular Configuration in Sodium Thymonucleate. Franklin R. and Gosling R.G" (PDF). Nature 171 (4356): 740–1. Bibcode 1953Natur.171..740F. doi:10.1038/171740a0. PMID 13054694. http://www.nature.com/nature/dna50/franklingosling.pdf.
- ↑ Wilkins M.H.F., A.R. Stokes A.R. & Wilson, H.R. (1953). "Molecular Structure of Deoxypentose Nucleic Acids" (PDF). Nature 171 (4356): 738–740. Bibcode 1953Natur.171..738W. doi:10.1038/171738a0. PMID 13054693. http://www.nature.com/nature/dna50/wilkins.pdf.
- ↑ Leslie AG, Arnott S, Chandrasekaran R, Ratliff RL (1980). "Polymorphism of DNA double helices". J. Mol. Biol. 143 (1): 49–72. doi:10.1016/0022-2836(80)90124-2. PMID 7441761.
- ↑ Baianu, I.C. (1980). "Structural Order and Partial Disorder in Biological systems". Bull. Math. Biol. 42 (4): 137–141. http://cogprints.org/3822/
- ↑ Hosemann R., Bagchi R.N., Direct analysis of diffraction by matter, North-Holland Publs., Amsterdam – New York, 1962.
- ↑ Baianu, I.C. (1978). "X-ray scattering by partially disordered membrane systems". Acta Crystallogr A 34 (5): 751–753. Bibcode 1978AcCrA..34..751B. doi:10.1107/S0567739478001540.
- ↑ Wahl M, Sundaralingam M (1997). "Crystal structures of A-DNA duplexes". Biopolymers 44 (1): 45–63. doi:10.1002/(SICI)1097-0282(1997)44:1<45::AID-BIP4>3.0.CO;2-#. PMID 9097733.
- ↑ Lu XJ, Shakked Z, Olson WK (2000). "A-form conformational motifs in ligand-bound DNA structures". J. Mol. Biol. 300 (4): 819–40. doi:10.1006/jmbi.2000.3690. PMID 10891271.
- ↑ Rothenburg S, Koch-Nolte F, Haag F (2001). "DNA methylation and Z-DNA formation as mediators of quantitative differences in the expression of alleles". Immunol Rev 184: 286–98. doi:10.1034/j.1600-065x.2001.1840125.x. PMID 12086319.
- ↑ Oh D, Kim Y, Rich A (2002). "Z-DNA-binding proteins can act as potent effectors of gene expression in vivo". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16666–71. Bibcode 2002PNAS...9916666O. doi:10.1073/pnas.262672699. PMC 139201. PMID 12486233. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139201.
- ↑ 41.0 41.1 "Arsenic-loving bacteria may help in hunt for alien life". BBC News. December 2, 2010. http://www.bbc.co.uk/news/science-environment-11886943. Retrieved 2010-12-02.
- ↑ 42.0 42.1 Bortman, Henry (2010-12-02). "Arsenic-Eating Bacteria Opens New Possibilities for Alien Life". Space.Com web site (Space.com). http://www.space.com/scienceastronomy/arsenic-bacteria-alien-life-101202.html. Retrieved 2010-12-02.
- ↑ "Arsenic-eating microbe may redefine chemistry of life". Nature News. 2 December 2010. http://www.nature.com/news/2010/101202/full/news.2010.645.html. Retrieved 2010-12-02.
- ↑ Greider C, Blackburn E (1985). "Identification of a specific telomere terminal transferase activity in Tetrahymena extracts". Cell 43 (2 Pt 1): 405–13. doi:10.1016/0092-8674(85)90170-9. PMID 3907856.
- ↑ Nugent C, Lundblad V (1998). "The telomerase reverse transcriptase: components and regulation". Genes Dev 12 (8): 1073–85. doi:10.1101/gad.12.8.1073. PMID 9553037.
- ↑ Wright W, Tesmer V, Huffman K, Levene S, Shay J (1997). "Normal human chromosomes have long G-rich telomeric overhangs at one end". Genes Dev 11 (21): 2801–9. doi:10.1101/gad.11.21.2801. PMC 316649. PMID 9353250. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=316649.
- ↑ Created from NDB UD0017
- ↑ 48.0 48.1 Burge S, Parkinson G, Hazel P, Todd A, Neidle S (2006). "Quadruplex DNA: sequence, topology and structure". Nucleic Acids Res 34 (19): 5402–15. doi:10.1093/nar/gkl655. PMC 1636468. PMID 17012276. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1636468.
- ↑ Parkinson G, Lee M, Neidle S (2002). "Crystal structure of parallel quadruplexes from human telomeric DNA". Nature 417 (6891): 876–80. doi:10.1038/nature755. PMID 12050675.
- ↑ Griffith J, Comeau L, Rosenfield S, Stansel R, Bianchi A, Moss H, de Lange T (1999). "Mammalian telomeres end in a large duplex loop". Cell 97 (4): 503–14. doi:10.1016/S0092-8674(00)80760-6. PMID 10338214.
- ↑ Seeman NC (2005). "DNA enables nanoscale control of the structure of matter". Q. Rev. Biophys. 38 (4): 363–71. doi:10.1017/S0033583505004087. PMID 16515737.
- ↑ Painter PC, Mosher LE, Rhoads C (1982). "Low-frequency modes in the Raman spectra of proteins". Biopolymers 21 (7): 1469–72. doi:10.1002/bip.360210715. PMID 7115900.
- ↑ Urabe H, Tominaga Y, Kubota K (1983). "Experimental evidence of collective vibrations in DNA double helix (Raman spectroscopy)". Journal of Chemical Physics 78 (10): 5937–5939. Bibcode 1983JChPh..78.5937U. doi:10.1063/1.444600.
- ↑ Chou KC (1984). "Low-frequency vibrations of DNA molecules". Biochem. J. 221 (1): 27–31. PMC 1143999. PMID 6466317. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1143999.
- ↑ Chou KC, Maggiora GM, Mao B (1989). "Quasi-continuum models of twist-like and accordion-like low-frequency motions in DNA". Biophys. J. 56 (2): 295–305. Bibcode 1989BpJ....56..295C. doi:10.1016/S0006-3495(89)82676-1. PMC 1280479. PMID 2775828. //www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1280479.
